
Training, guidelines and enforcement are lagging

Training, guidelines and enforcement are lagging

Agency plans to issue 'not yet classified' recall notices

TraceLink will oppose the motion


Move unites pharma efforts with larger healthcare network

$5-million settlement resolves six-year-old issue

Washington Post and 60 Minutes question McKesson’s 2017 $150-million fine

IQVIA’s eighth annual survey shows 77% of respondents go beyond compliance reporting

The Netherlands wins out in the competition to move the agency from London
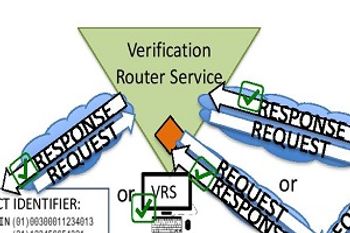
Wholesaler members ready themselves for DSCA-compliant returns processing in 2019

Counterfeit opioids and fake medical devices were a focus in the annual take-down of illicit distributors

Better accounting of sample distribution is offered

TraceLink charges HDA with antitrust activity over its Origin database

Intent of the 2016 Ensuring Patient Access and Effective Drug Enforcement Act is questioned

The Sharing Conference returns to Baltimore in 2017

PDUFA reauthorization creates new pathways; GAO report notes FDA’s 99% acceptance rate for expanded access

Master data on prescribers is combined with tracking expenditures, for transparency reporting

An estimated 64,070 deaths recorded; meanwhile, private equity flows into addiction centers
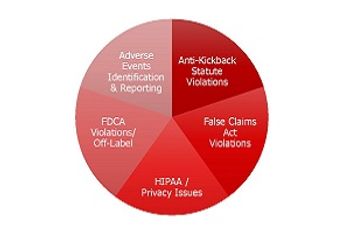
Pharma needs better internal policies to manage its increasing patient interaction

In the midst of the opioid crisis, drug distributors need to address DEA oversight
Observers are critical of the $465-million penalty as too lenient

New location, following Brexit, will be decided in November

Meanwhile, opioid manufacturers and distributors contend with multiple DEA penalties

Site lists manufacturers’ policies and criteria for experimental access

95% of consumers don’t know about online pharmacy certification